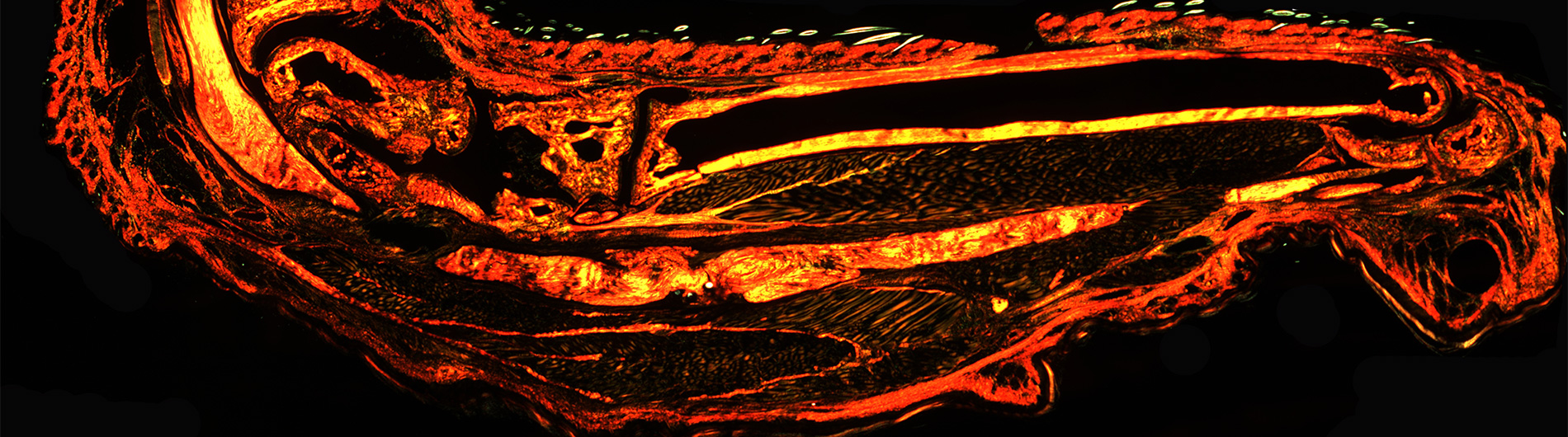
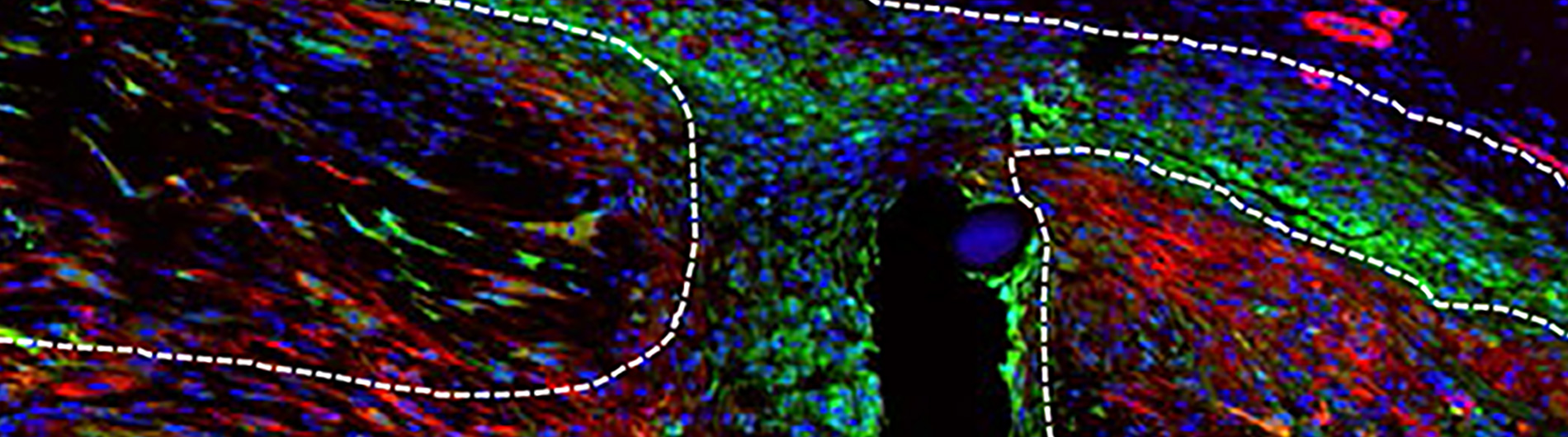
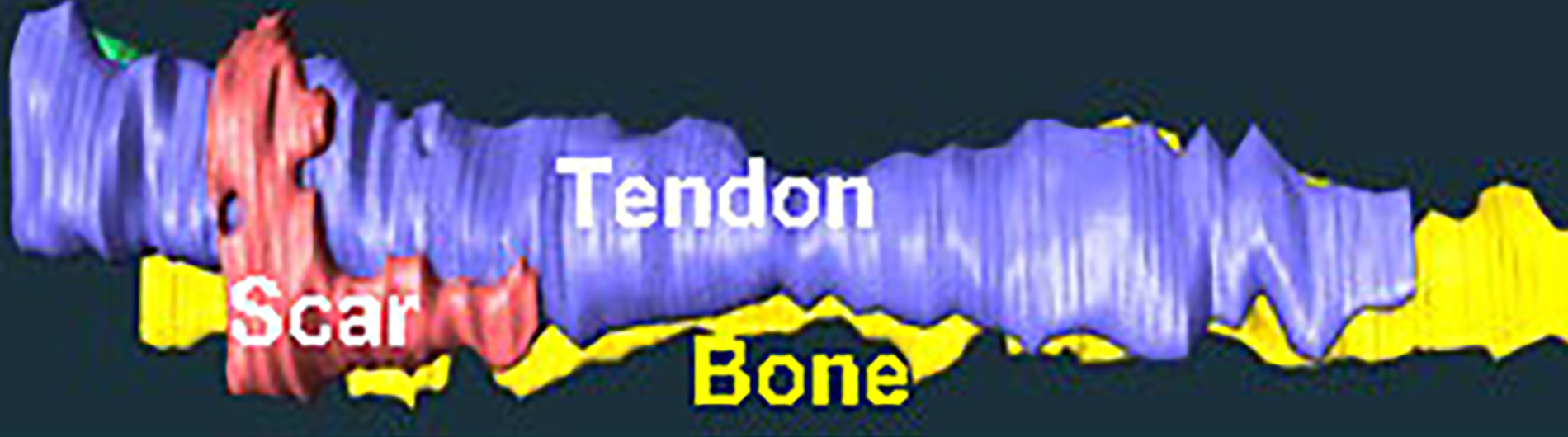
The overarching goals of our research program are to: i) identify novel strategies to promote regenerative tendon healing; ii) develop strategies to preserve tendon structure-function through the lifespan; and iii) understand the cellular and molecular drivers of soft tissue fibrosis, with a particular emphasis on the musculoskeletal system.

Alayna E. Loiselle, Ph.D.
Principal Investigator
Projects
View All ProjectsPublications
Defining the spatial-molecular map of fibrotic tendon healing and the drivers of Scleraxis-lineage cell fate and function. Ackerman JE, Best KT, Muscat SN, Pritchett EM, Nichols AEC, Wu C, Loiselle AE. Cell Reports. 2022 Nov 22. 41(8).
Scleraxis-lineage cell depletion improves tendon healing and disrupts adult tendon homeostasis. Best KT, Korcari A, Mora KE, Nichols AE, Muscat SN, Knapp E, Buckley MR, Loiselle AE. Elife. 2021 Jan 22;10:e62203. doi: 10.7554/eLife.62203. PMID: 33480357.
NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival. Best KT, Nichols AEC, Knapp E, Hammert WC, Ketonis C, Jonason JH, Awad HA, Loiselle AE. Sci Signal. 2020 Nov 17;13(658):eabb7209. doi: 10.1126/scisignal.abb7209. PMID: 33203721.
Cell non-autonomous functions of S100a4 drive fibrotic tendon healing. Ackerman JE, Nichols AE, Studentsova V, Best KT, Knapp E, Loiselle AE. Elife. 2019 May 24;8:e45342. doi: 10.7554/eLife.45342. PMID: 31124787.
View All PublicationsFebruary 11, 2022
URMC Researchers Reflect on International Day of Women and Girls in Science
January 31, 2022
2022 Kappa Delta Young Investigator Award Presented to Alayna E. Loiselle, PhD
July 28, 2021
$2M Grant Supports CMSR Study of Biologic Factors in Tendon Healing
March 1, 2020
Strong CMSR Representation at the 2020 ORS Meeting
Contact Us
Loiselle Lab
MC 1-8834
601 Elmwood Ave
Rochester, NY 14642