Research Programs

Artificial Intelligence / Machine Learning (AI/ML)
Artificial intelligence (AI) makes it possible for machines to simulate operations analogous to human learning, reasoning and self correction by analyzing massive amounts of data, allowing these systems to adjust to new inputs and perform human-like tasks. The artificial intelligence and machine learning program leverages neural networks and advanced custom models to perform automated tasks and address problems in musculoskeletal research which would otherwise be prohibitively complex or time-consuming. Major areas of investigation include automated analysis of ultrasound images and segmentation of areas of interest such as tendon scar formation or detection of major nerves leading to the development of new generation of diagnostic tools, use of machine vision to analyze lymphatic pathways shedding new light in the pathogenesis of rheumatoid arthritis and radiographic analysis of hand radiographs as a novel way to infer the presence of osteopenia or osteoporosis. We also undertake linguistic problems leveraging Natural Language Processing algorithms which allow rapid stratification of hundreds of thousands of patient messages based on perceived urgency and topic to better triage and appropriately divert communication.
Program Faculty: Warren Hammert, Constantinos Ketonis, Alayna Loiselle, David Mitten, Edward Schwarz
Bone Biology and Disease
Our Bone Biology and Disease program is focused on defining the signals and mechanisms important for bone formation and resorption in both normal and pathological situations (osteoporosis and osteopetrosis). While it is known that osteoblasts differentiate and localize the formation of new bone to regions where osteoclastic bone resorption has occurred, the signals responsible for this coupling effect are unknown and have become a focus of this research program. Another major research area in this program is centered on the pathophysiology of bone erosions in inflammatory arthritis, wear debris-induced osteolysis, which is responsible for aseptic loosening of orthopaedic implants and bone infections, or osteomyelitis. We have also made major advances in understanding how environmental hazards/toxins, such as lead and smoke, can predispose individuals to bone loss or osteoporosis.
Program Faculty: Brendan Boyce, Laura Calvi, Roman Eliseev, J. Edward Puzas, Edward M. Schwarz, Lianping Xing

Bone Cancer Biology
Cancers that involve the bone include primary tumors such as osteosarcoma, leukemias, and lymphomas that develop in the bone marrow and metastases of solid tumors including breast and prostate cancer. Involvement of the bone during cancer can lead to severe bone destruction, as well as treatment resistance and poor clinical outcomes. The Bone Cancer Biology research program is investigating these research questions with a basic biology approach to identify mechanisms of bone involvement in cancer and also with a translational approach to develop mechanistic insights into improved therapies in the clinic. Major areas of investigation in bone cancer biology include molecular mechanisms of bone marrow disruption in leukemia to be developed as therapeutic targets, improved targeting of therapy to the bone and bone marrow through nanoparticle and biochemical approaches, and screening biomarkers of renal cell carcinoma bone metastasis and understanding their roles, especially focused on tumor angiogenesis for potential therapeutic targets.
Program Faculty: Danielle Benoit, Laura Calvi, Roman Eliseev, Benjamin Frisch, J. Edward Puzas, Chao Xie, Lianping Xing
Cartilage Biology and Arthritis
Our Cartilage Biology and Arthritis program investigates the mechanisms of chondrogenesis, chondrocyte maturation, and chondrocyte metabolism during normal skeletal growth and cartilage disease. A major emphasis of this program is geared toward using genetic and injury-induced animal models to uncover the pathologic processes associated with inflammatory and non-inflammatory arthritis including: rheumatoid arthritis, psoriatic arthritis, lupus arthritis, and osteoarthritis. Our belief is that combining laboratory research with clinical investigation is the most effective way to bring better treatments to people affected by all types of arthritic disease.
Program Faculty: Brendan Boyce, Jennifer Jonason, Amy Lerner, Robert Mooney,
Christopher Ritchlin, Randy Rosier, Edward M. Schwarz, Lianping Xing, Xinping Zhang, Michael J. Zuscik
Drug Delivery
Continued understanding of mechanisms of musculoskeletal disease opens up opportunities for therapeutic development to treat disease or improve tissue regeneration. However, drug delivery to musculoskeletal tissues suffers from poor tissue biodistribution. For example, <1% of injected dose of small molecule drugs reaches bone tissue, and avascular cartilage has even greater delivery challenges. Thus, the drug delivery program exploits expertise in medicinal chemistry, nanotechnology, tissue targeting, and musculoskeletal and cancer biology to address musculoskeletal drug delivery hurdles.
Program Faculty: Hani Awad, Danielle Benoit, Brendan Boyce, Laura Calvi, Benjamin Frisch, J. Edward Puzas, Lianping Xing, Zhenqiang Yao
Fracture Repair and Bone Tissue Engineering
Skeletal injury is a significant health burden. By integration of various molecular, cellular, genetic, imaging, and engineering approaches, our musculoskeletal repair and maintenance program addresses challenges encountered by physicians when treating a wide array of musculoskeletal related injuries, namely large segmental defect, aging, and compromised skeletal healing. Animal models of fracture healing, structural bone grafting, distraction osteogenesis, and segmental and cranial bone defect healing models have been developed to study skeletal repair mechanisms. Major areas of investigation include 1) tissue engineering of multifunctional, periosteum mimetics for reconstruction of large bone defects; 2) understanding PTH effects of craniofacial bone defect healing; 3) investigating revascularization and repair of cranial bone grafts via intravital imaging; 4) developing noninvasive imaging modalities to assess graft vascularization and predict healing of tissue engineering approaches, and 5) integrating these signals into osteoconductive and osteoinductive 3D Printed Scaffolds.
Program Faculty: Hani Awad, Danielle Benoit, Alayna Loiselle, Edward M. Schwarz, Lianping Xing, Xinping Zhang, Michael J. Zuscik
Muscle Biology
Sarcopenia is the accelerated loss of skeletal muscle mass and function observed in most members of the elderly population and is a significant contributor to falls, frailty, and loss in functional mobility. Disabilities related to sarcopenia are a burgeoning cost to the US healthcare system. Additionally, the progression of sarcopenia correlates with reductions in neuromuscular junctions (NMJs) and the number and function of resident stem cells of skeletal muscle (satellite cells, SCs), which are also critically involved in the repair of injured muscle. To further understand the role of satellite cells in sarcopenia, NMJ, and skeletal muscle repair, the CMSR has research programs that utilize targeted mouse genetics and injury models to determine how molecules of interest affect satellite cell fate and skeletal muscle regenerative outcomes. Other work includes understanding the biological mechanisms of cancer-related skeletal muscle wasting and musculoskeletal injury prevention and performance enhancement.
Program Faculty: Calvin Cole, Robert Dirksen, Richard Moxley, Charles Thornton
Musculoskeletal Development
Our Musculoskeletal Development program is focused on identifying the mechanisms that underlie multiple aspects of axial and appendicular skeletal and skeletal muscle development including: patterning events, chondrogenesis, myogenesis, endochondral and intramembranous bone development, and joint formation. Developmental studies using genetic mouse models and primary cell culture techniques have identified multiple signaling molecules and transcription factors that are not only critical for normal musculoskeletal development, but are also implicated in congenital pediatric musculoskeletal disorders and adult musculoskeletal diseases.
Program Faculty: Jennifer Jonason, James Sanders, Michael Zuscik
Musculoskeletal Infection
Musculoskeletal infection is the bane of orthopaedic surgery and is associated with very poor clinical outcomes and crippling healthcare cost. To address this, the CMSR has active research projects aimed at elucidating the natural history of Staphylococcus aureus bone infection and biofilm formation and the etiology of co-morbidities (i.e. obesity, diabetes, aging) and developing novel diagnostics and interventions. The breadth of this research spans proof of concept research performed in a murine osteomyelitis model, through IND enabling studies in a novel clinically relevant sheep model of 2-stage exchange of an infected tibial implant with monoclonal antibodies (mAb) produced by our trainees in our cGMP facility. Additional projects involve the study of Type I and Type II diabetes in a murine osteomyelitis model; a world-wide Staphylococcal bone infection registry of serum and clinical isolates from 400 osteomyelitis patients; development of a custom 3D-printed antibiotic-impregnated calcium phosphate/collagen spacer evaluated in the murine osteomyelitis model; development of a 14-antigen Luminex assay as a serum diagnostic for S. aureus infection; and investigation of Staphylococcal biofilm formation on stainless steel and titanium implants in bone.
Program Faculty: Edward Schwarz, Hani Awad, Robert Mooney, Karen L. Bentley
Musculoskeletal Stem Cell Biology
Our Musculoskeletal Stem Cell Biology program covers broad interests in the identification, self-renewal, maintenance, cell fate determination, and differentiation of several types of musculoskeletal stem cells. These include mesenchymal stem cells that give rise to cartilage, bone, fat, and connective tissues, hematopoietic stem cells that generate all blood cells and are housed in the bone marrow, and skeletal muscle stem cells that are required for skeletal muscle growth and regeneration. We study these stem cells both in the context of embryonic development and adult musculoskeletal repair and tissue engineering. We are attempting to gain a broader understanding of the molecular circuits that regulate stem cell self-renewal and differentiation so that we may develop strategies to manipulate musculoskeletal stem cells for treatments of congenital skeletal dysplasias, age-related skeletal diseases (osteoporosis and osteoarthritis), bone fractures, myelodysplasias, sarcopenia, neuromuscular degenerative disorders, and skeletal and hematopoietic related cancers.
Program Faculty: Hani Awad, Danielle Benoit, Brendan Boyce, Laura Calvi, Roman Eliseev, Jennifer Jonason, Alayna Loiselle, J. Edward Puzas, Edward M. Schwarz, Lianping Xing, Xinping Zhang

Population Health
The Population Health Research Program is focused on improving the quality and lowering the costs of care for patients with osteoarthritis, rheumatoid arthritis, systemic lupus erythematosus, and several other musculoskeletal conditions. The goal of the Program is to generate evidence-based knowledge that can be used to optimize the value of care for patients, providers, and payers. Major areas of investigation in this Program include 1) the use of National Institutes of Health’s Patient-Reported Outcomes Measurement Information System for evaluating effectiveness of surgeries such as arthroplasties and spinal fusions; 2) implementation of The Center for Human Athleticism and Musculoskeletal Performance and Prevention (CHAMPP) program to improve sports performance, lifestyle choices, and academic outcomes for adolescent athletes and students who have been medically diagnosed as overweight/obese in Rochester’s inner-city and suburban schools; 3) examining the determinants of and variation in surgical outcomes such as complications and readmissions; and 4) evaluating the impact of Medicare’s payment reforms, such as the Bundled Payments for Care Improvement and the Comprehensive Care for Joint Replacement, on the processes and outcomes of musculoskeletal care.
Program Faculty: Allen Anandarajah, Calvin Cole, William Hammert, Michael Maloney, Emmanuel Menga, Addisu Mesfin, Benjamin Ricciardi, Katherine Rizzone, Paul Rubery, James Sanders, Ilya Voloshin
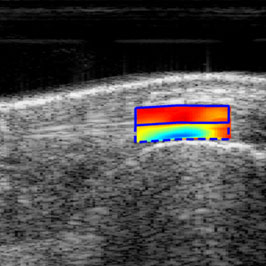
Tendon Development, Repair and Regenerative Engineering
Tendons connect muscle to bone to maintain the musculoskeletal posture and effect motion. Maintaining or restoring proper tendon function is critical to musculoskeletal health. Using multi-disciplinary approaches, the tendon research program focuses on a broad spectrum of research questions from fundamental cell, molecular, and development biology to clinical application. Major areas of investigation include identifying mechanisms to promote regenerative healing using multi-omics and developmental biology to inform tissue engineering, including biomaterials and nanomedicine approaches; understanding fundamental aspects of tendon biomechanics and mechanobiology; and the development of non-invasive imaging approaches to assess tendon function and healing.
Program Faculty: Hani Awad, Danielle Benoit, Mark Buckley, Constantinos Ketonis, Alayna Loiselle