News
GDSC Fall Retreat 2019
Wednesday, November 20, 2019

Last month, on Friday October 25th, the GDSC celebrated our annual Fall Retreat at the Olmsted Lodge in Highland Park. Among our numerous reasons to celebrate 2019 are our two most recent faculty additions, Isaac Harris and Jeevisha Baja. Our festivities began with a round of pumpkin carvings for kids, and those of us who are still kids at heart. The night continued with a game of faculty trivia. GDSC student, Anne Roskowski earned first place with a perfect score! Our games concluded with a history of science themed game of Jeopardy. Throughout the evening attendees exchanged their home cooking and recipes. We thank everyone who enriched the night with their cuisine. We also appreciate the efforts of our Fall Retreat Committee; 1st year GDSC students Emily Berry, Jamie Burchett, Dakarai Esgdaille, and Ludia Pack.

GDSC Halloween Costume Contest!
Wednesday, November 20, 2019
Last month the GDSC held our Halloween Costume Contest. Our four contestants can be seen below.
The votes are in and the winner is the Mello Lab as the cast from Alice in Wonderland! Thank you to all GDSC costume participants.

The Mello Lab as the Cast from Alice In Wonderland

Maria Lazaro-Pena, as a Ghost

Sara Lolo, as Hercules

Anne Roskowski, as My Fair Lady
24th Annual WCI Scientific Symposium
Thursday, November 14, 2019

On Thursday, November 14 the GDSC supported the Wilmot Cancer Institute (WCI) in hosting their Twenty Fourth Scientific Symposium for Cancer Research and Treatment. At symposium's poster session students from the GDSC and across the UR, working in basic, translational and clinical cancer research showcased their respective cancer studies in the Flaum Atrium.
 This year's poster winners were:
This year's poster winners were:
- Amber S. Keckner
- Jacob G Kallenbach
- Nicholas G. Battaglia
- Taylor P. Uccello
- Amelia M. Clark from Brian Altman's Lab
This year's Grand Poster Winners were:
- Marian Ackun-Farmmer
- Andrea M. Amitrano
Symposium speakers included GDSC faculty Isaac S. Harris and Jeevisha Baja. WCI professor Edith Lord, gave the Davey Award Lecture titled Radiotherapy and the immune response in the tumor microenvironment.
Finally, this year's Underberg Lecturer was Ari M. Melnick, M.D. Gebroe Family Professor of Hematology/Oncology from Cornell University. His lecture was titled Precision Epigenetic Therapy for B-Cell Lymphomas.


Welcome to the Class of 2019 GDSC Students
Thursday, September 19, 2019
September marks the start of graduate life in earnest for our incoming class of students: Emily Berry ('19, Univ. of New Hampshire), Jamie Burchett ('19, Northern Kentucky Univ.) Dakarai Esgdaille ('19 Wells College), Ludia Pack ('19 Univ. of Rochester) and Brandon Park ('19, Penn State Univ.). And all five students are off to a strong start:
Emily Berry was awarded the Merritt and Marjorie Cleveland Fellowship award, Ludia Pack received the 2019 J. Newell Stannard Graduate Student Scholarship Award, Dakarai Esgdaille was nominated for Graduate Alumni Fellowship Award and Jamie Burchett was an Irving L. Spar Fellowship nominee. Congratulations to all! Also a warm welcome, and best of luck as you embark on your new career paths!

Emily Berry

Jamie Burchett

Dakarai Esgdaille

Ludia Pack

Brandon Park
GDSC Team makes a strong showing at the 7th annual Wilmot “Warrior Walk”
Wednesday, September 11, 2019

Students and faculty from Biomedical Genetics and the GDSC graduate program supported the 7th Wilmot Cancer Institute Warrior Walk last Sunday. Team "GDSC Cancer Warriors" symbolized the graduate students, post-docs and faculty working hard to find new approaches to defeating cancer - and that is in addition to the over $1000 raised by the team. A big thank you to all who gave their time and money to directly support our cancer patients and survivors!
In addition to the Survivor Walk, "GDSC Cancer Warriors" also participated in the 10K and 5K events. Notably, Patrick Murphy (10K) placed 4th, Brian Altman (5K) placed 5th, Fanju Meng (5K) placed 8th, Isaac Harris (5K) placed 15th and Xiaolu Wei placed 18th in their age-groups. Congrats on a great effort all around!
Leigh Wexler Graduates!
Friday, September 6, 2019

Dr. Leigh Wexler and Dr. Portman (From Left)
Congratulations to Dr. Leigh Wexler, who successfully defended their thesis this week, earning a Ph.D. in Genetics from the GDSC program. Leigh's thesis research in the Portman Lab focused on the regulation of neuronal circuit function and behavior in the nematode C. elegans. It's been known for many years that males of this species tend to leave a food source to find mates, but that depriving males of food causes them to reprioritize feeding behavior over exploration. One important component of this behavioral flexibility is regulated chemosensory function. Well-fed males detect food poorly, partly due to low expression of a food-associated chemoreceptor called ODR-10, but food-deprived males upregulate ODR-10, increasing food attraction and decreasing food-leaving behavior. In contrast, hermaphrodites (the female equivalent in C. elegans) are strongly attracted to food and exhibit high levels of ODR-10 expression even when well-fed.
Leigh's research probed the mechanism by which ODR-10 expression is influenced by feeding status in males. They found that signals through two conserved pathways, involving the TGFβ-family ligand DAF-7 and the insulin-like (IIS) receptor DAF-2, are important for keeping ODR-10 expression low in well-fed males. Further, Leigh found that males in which the IIS pathway is constitutively active fail to upregulate ODR-10 when starved. Interestingly, the DAF-7 signal appears to act upstream of IIS, indicating that a cascade of neuroendocrine interactions is necessary for repressing ODR-10. And DAF-7 does not act as a sensor of the starved male's physiological state, but rather conveys information about the presence of food in the environment.
Together, Leigh's research demonstrates that C. elegans males assess their external state, rather than their metabolism, when deciding whether to take the risk of leaving food to find a mate, and that this occurs through a multistep neuroendocrine feedback loop. Leigh's work also provides important insights into how internal and external states are integrated by the nervous system to influence gene expression, neuronal circuit function, and behavior. This work will appear in an upcoming issue of Current Biology. We wish Leigh all the best as she set out to Boston, to start her post-doctoral career in the laboratory of Max Heiman at Harvard.
Congratulations Dalia Ghoneim!
Tuesday, July 23, 2019

On July 19, Dalia Ghoneim earned her Ph.D. for successfully defending her thesis, "New functions for RNA elucidated by evolutionary conservation." She demonstrated that adjacent pairs of codons known to inhibit protein expression and to slow translation in yeast are conserved, rather than avoided. Genes with these conserved codon pairs exhibit altered translation properties. Thus, conservation is evidence that these codon pairs serve a function in yeast, although that function is yet to be determined. Additionally, in collaboration with Xin Li's lab, Dalia scanned mouse sperm transcriptome data using a machine learning method to identify sets of long-non-coding RNAs with conserved structures. Dalia was mentored by David H. Mathews and Beth Grayhack. During her studies, Dalia was awarded the prestigious Perricone MD Born Seekers fellowship. We wish Dalia all the best for her post-doctoral career!

Congratulations MJ
Thursday, July 11, 2019
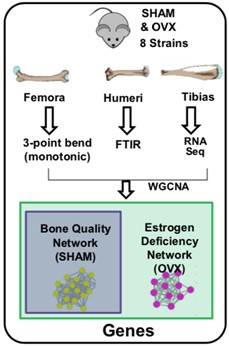 After successfully defending his thesis, MJ has completed the PhD portion of his MD/PhD studies. MJ's studies investigated the genetic basis of bone-matrix quality, an underappreciated property of bone that contributes significantly to bone strength and is of great clinical importance for understanding of bone pathology such as osteoporosis. MJ used both a classical population genetics approach as well as a systems genetics approach, and found that bone matrix characteristics such as morphology and matrix composition are indeed inheritable properties. In addition, using an estrogen-deficient model of post-menopausal bone loss, he was able to identify gene networks that may play an important role in osteoporosis. His results suggest that bone matrix quality is influenced by genetics and participates in maintaining tissue-level mechanical properties. Furthermore, identifying putative regulatory genes is clinically significant as they are presumptive targets for developing novel therapeutics. During his thesis work, MJ was the recipient of a CTSI Pilot Trainee Grant and scored a fundable F31 predoctoral fellowship from NIAMS. With his GDSC doctorate in hand MJ will return to medical school to obtain his M.D. Best of luck in your future ventures, MJ!!
After successfully defending his thesis, MJ has completed the PhD portion of his MD/PhD studies. MJ's studies investigated the genetic basis of bone-matrix quality, an underappreciated property of bone that contributes significantly to bone strength and is of great clinical importance for understanding of bone pathology such as osteoporosis. MJ used both a classical population genetics approach as well as a systems genetics approach, and found that bone matrix characteristics such as morphology and matrix composition are indeed inheritable properties. In addition, using an estrogen-deficient model of post-menopausal bone loss, he was able to identify gene networks that may play an important role in osteoporosis. His results suggest that bone matrix quality is influenced by genetics and participates in maintaining tissue-level mechanical properties. Furthermore, identifying putative regulatory genes is clinically significant as they are presumptive targets for developing novel therapeutics. During his thesis work, MJ was the recipient of a CTSI Pilot Trainee Grant and scored a fundable F31 predoctoral fellowship from NIAMS. With his GDSC doctorate in hand MJ will return to medical school to obtain his M.D. Best of luck in your future ventures, MJ!!
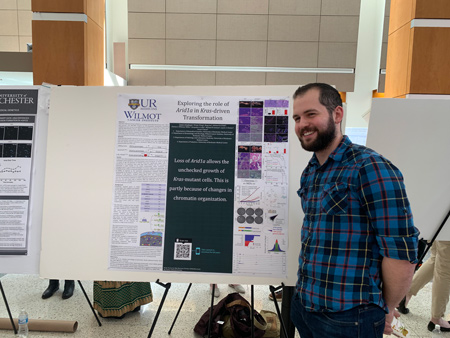
Scott Friedland Defends Thesis
Thursday, July 11, 2019
This week M.D./Ph.D. student Scott Friedland defended his doctoral thesis. Arriving at the GDSC in 2014, Scott pursued his Ph.D. under the mentorship of Dr. Aram Hezel. As a student Scott was granted travel awards to a national physician scientist conference and selective course at Cold Spring Harbor Labs. His thesis, titled Arid1a, a subunit of the SWI/SNF chromatin remodeling complex, is a barrier to KrasG12D-driven tumorigenesis, studied the role of SWI/SNF, a chromatin remodeling complex, in pancreatic function and disease, which has implications for the fields of cancer and developmental biology. These findings may, in time, impact the treatment of diseases such as pancreatitis and pancreatic cancer. Scott will be reinitiating his medical education alongside the class of 2021 here at the University of Rochester School of Medicine, and is interested in pursuing a career as an oncologist and cancer researcher. Congratulations Dr. Scott Carl Friedland!
Studies Led by Douglas Portman Examine Nervous System Changes During Puberty
Tuesday, July 9, 2019
Very little is known about how the onset of puberty is controlled in humans, but the discovery of a new gene in the roundworm C. elegans could be the "missing link" that determines when it's time to make this juvenile-to-adult transition. Two genes, LIN28and MKRN3, are known to be associated with precocious puberty in humans, where juveniles as young as six may start developing adult features. These genes are found in all animals, including C. elegans, in which they also control the juvenile-to-adult transition. Until the new discovery, it was unclear how these two genes are connected.
The more obvious signs of the transition of juvenile-to-adult tend to be external—body morphology, matured genitalia—but nervous system changes are also happening at the same time. In humans, the maturation of the brain during adolescence is associated with increased vulnerability to a variety of neuropsychiatric disorders, so a better understanding of these processes is important for understanding mental health as well as basic neurobiology.
Two new studies in the labs of Douglas Portman, Ph.D. at the University of Rochester Medical Center and David Fitch at New York University, published in Developmental Cell and eLife, identified a new developmental timing mechanism involving a long non-coding RNA in the microscopic roundworm C. elegans. Their research revealed a surprising new molecular mechanism that controls the timing of sex-specific changes in body shape, the maturation of neural circuits, and behavior.
C. elegans has long been used by researchers to understand fundamental mechanisms in biology. Many of the discoveries made using these worms apply throughout the animal kingdom and this research has led to a broader understanding of human biology. In fact, three Nobel Prizes in medicine and chemistry have been awarded for discoveries involving C. elegans.
The researchers identified a new gene that, when disrupted, delays the transition from the juvenile to the adult stage. Surprisingly, this gene, called lep-5, does not act as a protein, as most genes do. Instead, it functions as a long non-coding RNA (lncRNA), a recently discovered class of genes whose functions remain largely mysterious. The team observed that this lncRNA is important for promoting the juvenile-to-adult transition by directly interacting with LIN-28 and LEP-2, a C. elegans gene similar to MKRN3. Because the human versions of LEP-2 and LIN-28 are both involved in the timing of puberty, the new research suggests that a yet-to-be-discovered lncRNA might be essential to this process in humans as well.
A Graphic Design Revolution For Scientific Conference Posters
Tuesday, June 18, 2019
"Other templates didn't necessarily ask you to think about what you were putting on them because they allowed it all," says Derek Crowe, a PhD student in biomedical genetics in Hucky Land's lab with a former career in visual communication and design, "In order to use Mike's layout though, my hand is forced."
With the new template, scientists need to think about their core message, but some people have a difficult time figuring out how to do that, or how to use visuals to present their message. Without proper science communication training, even a better poster template doesn't work.
Crowe has taken matters into his own hands. Not only does he teach a course on visual communication for scientists at the University of Rochester, but he also shared his poster design tips online. In a nod to Morrison's "better poster", Crowe's is a "butter poster". He provides step by step instructions on how to organize the poster, and how to think about the content in a visual way.
"Like the graphic novel did for literature, visual languages have the power to add more dimensions to scientific storytelling," says Crowe, "I'm excited to see what happens as the greater science community begins to take advantage of well-established visual storytelling tools."
GDSC Student, Tom O’Connor Earns First Place in 2019 Sharing Your Science in A Social World Contest.
Monday, June 3, 2019
Finishing his first year of GDSC studies on a high-note, Tom O'Connor (member of the Chakkalakal & Dirksen Labs) together with his teammate Griffin Schroeder, have won first place in the 2019 'Sharing Your Science in A Social World' contest. Sponsored by URBEST, this contest encourages students to communicate their research to a broad audience using videography. For their entry, imbedded above, Tom and Griffin emphasized the work being done in the Noble Lab, where Tom worked during his first rotation. The video, URBEST 2019: Translational Science, highlights how the Noble Lab sets itself apart by re-purposing FDA approved drugs for different clinical applications, expediting the bench-to-clinic transition.
Congratulations Tom and Griffin!
Next-Gen Women in Science: Dalia Ghoneim
Thursday, May 2, 2019


GDSC student Dalia Ghoneim from the Matthews lab was awarded the prestigious Perricone MD Born Seekers fellowship. The $20,000 award recognizes the inspiring achievements of young women in science, and is the culmination of the Scientista Foundation's video competition, in which young women tell their personal journey in STEM. Dalia was able to tell her remarkable story in the award-winning video clip with the help of two talented fellow GDSC-students: cameraman, sound expert and producer Adam Cornwell, and speech-editor Matt Ingalls. As a single mother of four, Dalia is now approaching the successful completion of her PhD in Genetics. She was invited to give her speech and accept her award at the Scientista Symposium 2019 in Boston, MA. The Scientista Foundation's vision is to support the next generation of female scientists -- and we can't wait to see what Dalia will do next! Congratulations!!
Jayme Olson earns (CTSI) Trainee Award
Tuesday, April 30, 2019
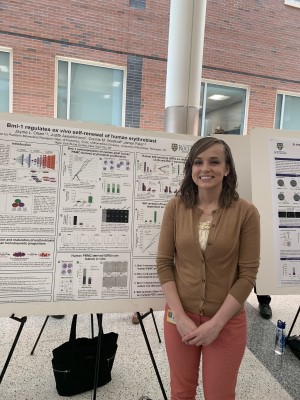 Jayme a GDSC-graduate student in the Palis Lab was recently awarded a Clinical and Translational Science Institute (CTSI) Trainee Award. This one-year fellowship will fund cutting edge work on the in vitro generation of human red blood cells. Cultured human red blood cells (RBCs) have the potential to serve as a supplemental source of blood for transfusion therapy, and as a tool for clinical and research diagnostic. However, a major barrier in generating sufficient numbers of cultured RBCs cells is the limited ex vivo self-renewal capacity of adult-derived erythroblasts. Work in the Palis Lab has identified Bmi-1, a member of the polycomb repressive complex 1 (PRC1), as a critical regulator of erythroid self-renewal. Bmi-1 also plays a role in normal erythroid precursor maturation. Jayme will test the hypothesis that Bmi-1 regulates erythroid self-renewal and terminal maturation using different PRC1 members. Ultimately, these proposed studies will pave the way for the generation of sufficient numbers of cultured RBCs for blood typing and transfusion therapy, as well as the establishment of in vitro models for the study of erythroid intrinsic diseases.
Jayme a GDSC-graduate student in the Palis Lab was recently awarded a Clinical and Translational Science Institute (CTSI) Trainee Award. This one-year fellowship will fund cutting edge work on the in vitro generation of human red blood cells. Cultured human red blood cells (RBCs) have the potential to serve as a supplemental source of blood for transfusion therapy, and as a tool for clinical and research diagnostic. However, a major barrier in generating sufficient numbers of cultured RBCs cells is the limited ex vivo self-renewal capacity of adult-derived erythroblasts. Work in the Palis Lab has identified Bmi-1, a member of the polycomb repressive complex 1 (PRC1), as a critical regulator of erythroid self-renewal. Bmi-1 also plays a role in normal erythroid precursor maturation. Jayme will test the hypothesis that Bmi-1 regulates erythroid self-renewal and terminal maturation using different PRC1 members. Ultimately, these proposed studies will pave the way for the generation of sufficient numbers of cultured RBCs for blood typing and transfusion therapy, as well as the establishment of in vitro models for the study of erythroid intrinsic diseases.
31st Genetics Day at the University of Rochester
Monday, April 29, 2019
 The University of Rochester hosted its 31st Annual Genetics Day Symposium with a poster session displaying genetics research from more than fifty post-doctoral fellows, graduate and undergraduate students. The meeting started with a strong lineup of faculty presentations, highlighting ongoing Genetics Research and as well as new faculty recruits. Speakers included Dr. Amanda Larracuente, Dr. Doug Anderson, Dr. Peng Yao, Dr. Xin Zhiguo Li and Dr. Paul Boutz. Keynote speaker Dr. Phillip Zamore, from the University of Massachusetts, delivered the 17th Annual Fred Sherman Lecture. This year's poster prizes were awarded to:
The University of Rochester hosted its 31st Annual Genetics Day Symposium with a poster session displaying genetics research from more than fifty post-doctoral fellows, graduate and undergraduate students. The meeting started with a strong lineup of faculty presentations, highlighting ongoing Genetics Research and as well as new faculty recruits. Speakers included Dr. Amanda Larracuente, Dr. Doug Anderson, Dr. Peng Yao, Dr. Xin Zhiguo Li and Dr. Paul Boutz. Keynote speaker Dr. Phillip Zamore, from the University of Massachusetts, delivered the 17th Annual Fred Sherman Lecture. This year's poster prizes were awarded to:
- Leigh Wexler: A male-specific neuroendocrine feedback loop couples food signals for feeding behavior in C. Elegans
- Matthew Tanner: Identifying sequence determinants of altered RNA splicing in myotonic dystrophy.
- Dr. Jacquelyn Lillis: Single-cell transcriptome analysis of embryonic erythro-myeloid progenitor cells reveals lineage heterogeneity.
- Jayme L. Olsen: Bmi-1 regulates human erythroblast ex vivo self-renewal.
- Anissa Elahi: Transglutaminase 2 as a therapeutic target to facilitate recovery after spinal cord injury.
- Zhengfen (Jeff) Liu: DNA damage-specific regulation of cell cycle checkpoint by γ-h2ax.
We congratulate each poster winner and look forward to the 32nd Genetics Day next year!
A link to additional Genetics Day 2019 photos can be found here.
Researchers Strike Key to Longevity
Sunday, April 28, 2019
Researchers at the University of Rochester have uncovered more evidence that the key to longevity resides instead in a gene, a Rael-Science post says.
Explorers for centuries have however, dreamt of a Fountain of Youth, with healing waters that rejuvenate the old and extend life indefinitely.
In a new paper published in the journal Cell, the researchers—including Vera Gorbunova and Andrei Seluanov, professors of biology; Dirk Bohmann, professor of biomedical genetics; and their team of students and postdoctoral researchers—found that the gene sirtuin 6 (SIRT6) is responsible for more efficient DNA repair in species with longer lifespans.
The research illuminates new targets for anti-aging interventions and could help prevent age-related diseases.
As humans and other mammals grow older, their DNA is increasingly prone to breaks, which can lead to gene rearrangements and mutations—hallmarks of cancer and aging. For that reason, researchers have long hypothesized that DNA repair plays an important role in determining an organism's lifespan.
While behaviors like smoking can exacerbate double-strand breaks (DSBs) in DNA, the breaks themselves are unavoidable. "They are always going to be there, even if you're super healthy," says Bohmann. "One of the main causes of DSBs is oxidative damage and, since we need oxygen to breathe, the breaks are inevitable."
Organisms like mice have a smaller chance of accumulating double-strand breaks in their comparatively short lives, versus organisms with longer lifespans, Bohmann says. "But, if you want to live for 50 years or so, there's more of a need to put a system into place to fix these breaks."
'Longevity Gene' That Helps Repair DNA And Extend Life Span Could One Day Prevent Age-Related Diseases In Humans
Tuesday, April 23, 2019
Scientists studying longevity believe a gene could explain why some animals live longer.
In 18 species of rodents with varying life spans, researchers looked at sirtuin 6 (SIRT6), a gene that plays a role in bodily processes such as aging, cellular stress resistance and DNA repair.
Over time, DNA inevitably suffers what are known as double-strand breaks (DSBs) that can cause genes to mutate, triggering aging and diseases like cancer.
Dirk Bohmann, a professor of biomedical genetics at the University of Rochester Medical Center, explained in a statement, "[DSBs] are always going to be there, even if you're super healthy. One of the main causes of DSBs is oxidative damage and, since we need oxygen to breathe, the breaks are inevitable."
While animals with relatively short life spans don't have so many DSBs, Bohmann explained, "if you want to live for 50 years or so, there's more of a need to put a system into place to fix these breaks. The SIRT6 protein seems to be the dominant determinant of lifespan. We show that at the cell level, the DNA repair works better, and at the organism level, there is an extended lifespan."
To answer whether SIRT6 works harder in species that live longer, the team studied 18 rodents, from mice expected to live around three years to beavers and mole rats with life expectancies of up to 32 years. Animals with stronger SIRT6 proteins were found to live longer.
This was also apparent when they compared the molecular differences in the SIRT6 proteins of mice and beavers. And by dosing human cells and fruit flies with the SIRT6 from a mouse and a beaver, as expected, the scientists found the beaver protein was more potent than the mouse protein.
31st Annual Genetics Day Symposium
Monday, April 22, 2019
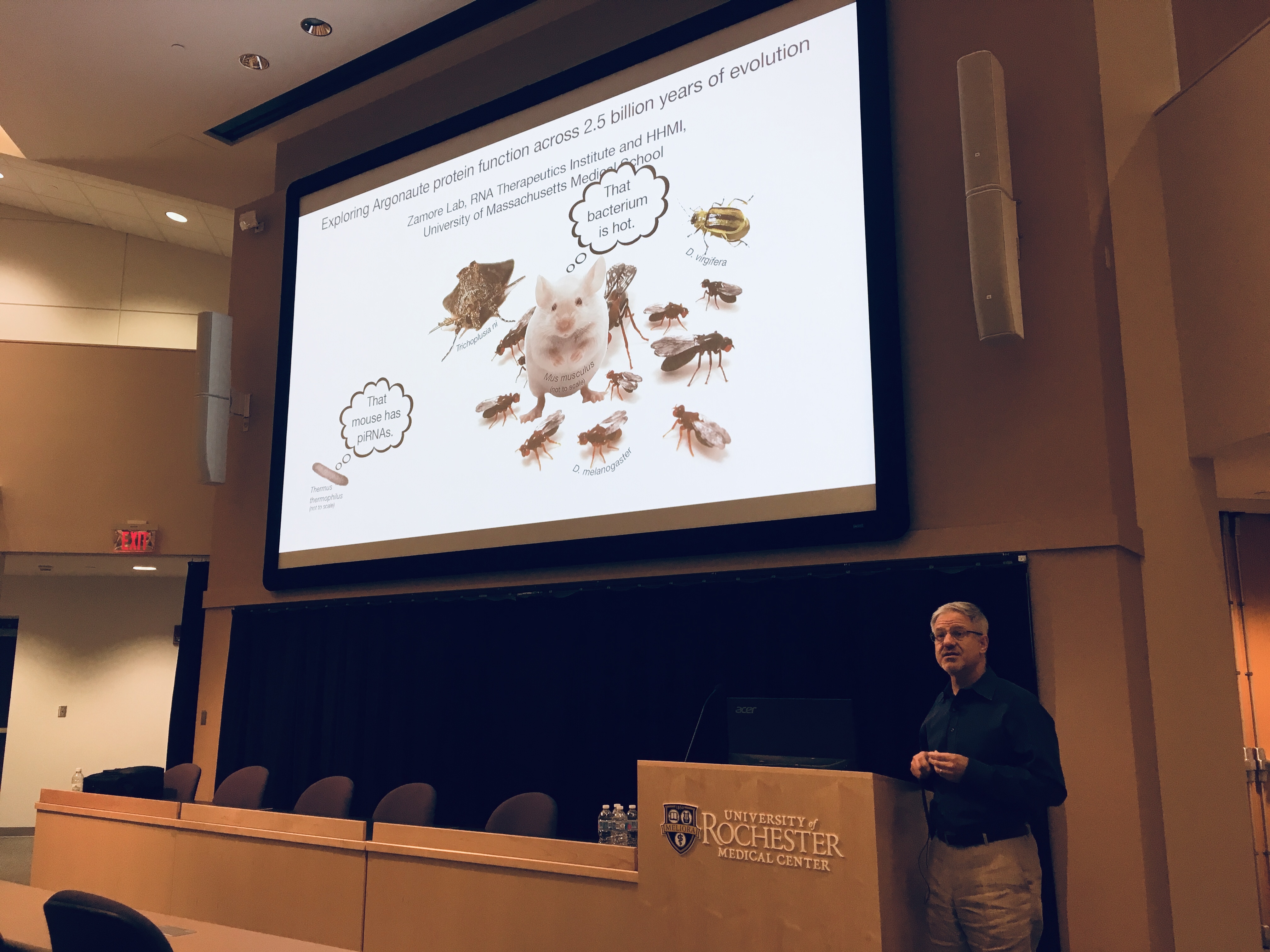
The Departments of Biomedical Genetics and Biology, with the support of the University Committee for Interdisciplinary Studies, host the 31st annual Genetics Day Symposium on Thursday, April 25, from 9:30 a.m. to 4 p.m. in the Class of '62 Auditorium and Flaum Atrium. This year's Fred Sherman Lecturer will be Phillip Zamore, a Howard Hughes Medical Institute investigator and professor of biomedical sciences at the University of Massachusetts Medical School, giving a talk titled "piRNAs and the Struggle to Reproduce."
Jared Mereness successfully defends thesis
Thursday, March 14, 2019

Last week, Jared Mereness successfully defended his PhD Thesis. Since arriving at the University of Rochester in 2012, Jared has worked with his advisor, Tom Mariani, and studied Lung and Pulmonary diseases. During his studies Jared was awarded an institutional T32 Training Grant in Pulmonary Research, and a Howard Hughes Medical Institute Med-Into-Grad Fellowship in Translational Cardiovascular Research at the University of Rochester. Jared's research and thesis has focused on describing the role of the extracellular matrix component, collagen 6, in lung structure, and its effects on the function of pulmonary epithelial cells. His findings broaden our understanding of potential roles this unique extracellular matrix protein may have in chronic lung disease and the development and maintenance of lung structure. Soon Jared will begin a Postdoc position in Danielle Benoit's laboratory in Biomedical Engineering at the University of Rochester. Congratulations Jared!
For further Reading, please see: PLOS ONE: Type VI collagen promotes lung epithelial cell spreading and wound-closure by Mereness et al.

Falsey, Mariani Secure $3.8 M NIH Grant to Reduce Antibiotic Overuse
Thursday, February 21, 2019
Ann R. Falsey, M.D., professor of Infectious Diseases, and Thomas J. Mariani, Ph.D., professor of Pediatrics, received a 5-year, $3.8 million grant from the National Institutes of Health to search for a better way to distinguish bacterial and viral respiratory infections. The goal of the study is to define predictive genes -- using gene expression profiling of blood -- that can be developed into a simple point of care diagnostic that can be used by clinicians to discriminate bacterial and non-bacterial illness. Such a test would allow physicians to optimally manage patients with acute respiratory infections, which are a leading cause of antibiotic overuse and are linked to the rise of antibiotic resistant organisms.
The grant is the result of research done as part of the NIH-funded Respiratory Pathogens Research Center. Falsey and Mariani are the co-principal investigators, and Edward Walsh, M.D.,Angela Branche, M.D. and Derick Peterson, Ph.D. are co-investigators.
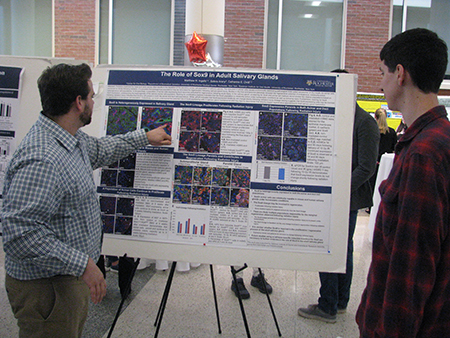
Matt Ingalls wins Prestigious Poster Prize at Gordon Conference
Friday, February 15, 2019
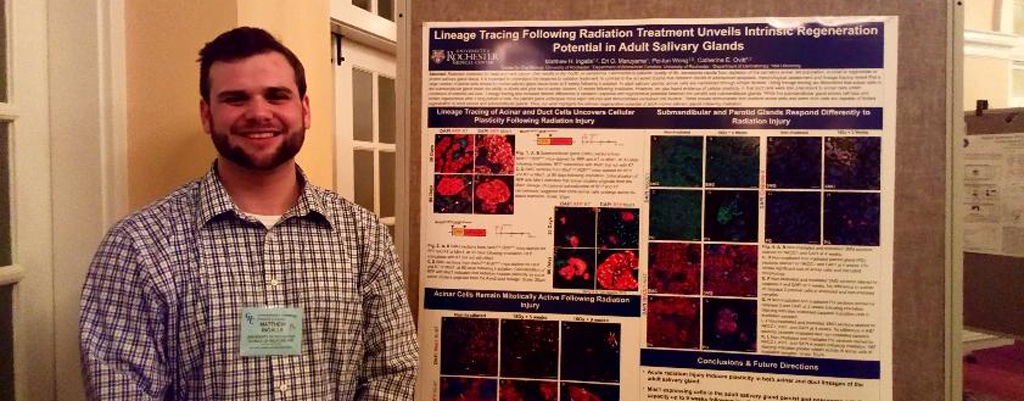
Matt Ingalls With Poster

Matt Ingalls with other award winners
GDSC student Matt Ingalls won an award for his poster presentation at the 2019 Gordon Research Conference for Salivary Glands and Exocrine Biology in Galveston, Texas (February 2nd -- 8th). The GRC brought together leading researchers in the field of salivary gland biology from around the world. Matt's poster, titled "Lineage Tracing Following Radiation Treatment Unveils Intrinsic Regeneration Potential in Adult Salivary Glands", highlights differences in radiation response between the submandibular and parotid salivary glands. Utilizing lineage tracing models his work demonstrates the intrinsic regeneration potential of the adult salivary gland. The NIH-supported research was conducted in the laboratory of Dr. Catherine Ovitt and was co-authored by E. Maruyama and P. Weng. -- Congratulations Matt!
GDSC student Adrian Molina-Vargas co-founds ADSE chapter to tackle underrepresentation in STEM
Friday, February 1, 2019
February 1, 2019

In the front row from the left, Keon Garrett, Ellen Matson, Raven Osborn, and Antonio Tinoco Valencia; and in the back row from the left, Marian Ackun-Farmmer, Heta Gandhi, Adrian Molina Vargas, Shukree Abdul-Rashed, and Liz Daniele are among the founding members of the new Rochester chapter of the Alliance for Diversity in Science and Engineering. (University of Rochester photo / J. Adam Fenster)
Raven Osborn thought long and hard about continuing a PhD at the University of Rochester. Other minority students she knew at the Medical Center had also felt the isolation, the constant "being on edge" and "code-switching"—shifting the way they express themselves—that comes with being an underrepresented minority in a STEM field.
"Can I do this for another five and half years?" she wondered.
Antonio Tinoco, a DREAMer who was born in Mexico and raised in Los Angeles, is a fourth year PhD student in the department of chemistry on the River Campus. He can remember only one or two occasions when a visiting faculty member of underrepresented minority background was invited to give a seminar in his department.
"My goal is to go into academia to be a professor, do research, and teach. But there are so few examples to follow," he says. "I don't even know of anyone who, as a DACA recipient or DREAMer, is a professor in chemistry. So, I could easily tell myself nobody has done it; it's impossible; maybe I should look for something else."
Instead, Tinoco, Osborn, and five other graduate students have banded together to form the University of Rochester chapter of the Alliance for Diversity in Science and Engineering (ADSE). The mission of the national ADSE, which was founded in 2014, is to increase the participation of underrepresented groups in academia, industry, and government through graduate student organizations that reach out to students and scientists of all ages and backgrounds.
Other ADSE chapters are at the University of California campuses at Berkeley and Davis, the University of Central Florida, the University of Colorado, Columbia University, Drexel University, Georgia Institute of Technology, University Maryland, New York University, Northeastern University, and Texas A&M.
Tinoco, the president and founding member of the new chapter, says its immediate goals are twofold:
- Establish a diversity lecture series to bring underrepresented faculty from other universities to Rochester. "It would be an opportunity for underrepresented minority students here to say 'Wow, there's someone out there like me who is making it, so maybe there's hope for me.'" Underrepresented minority postdoctoral fellows would also be invited, especially ones who might be interested in eventually teaching here, Tinoco says.
- Provide a space where underrepresented graduate students in STEM fields from across the University can meet, network, and hold workshops and panels to openly discuss the issues they face. "If we can openly discuss these things, we won't feel as isolated," Tinoco says.
The chapter has been certified by the University and will receive funding through the University's David T. Kearns Center for Leadership and Diversity. ADSE's goals fall well within the Kearns Center's mission to expand the educational pipeline through the doctoral degree for low-income, first-generation college, and underrepresented minority students, says Liz Daniele, the center's assistant director for graduate diversity.
Inviting underrepresented faculty from other campuses to give a science-based talk, but also give a diversity-themed talk about their academic journey "is a great model," she says. "And that's why Kearns is happy to support several semesters of lectures."
"I think this is exactly the type of thing that the University needs right now," says Ellen Matson, assistant professor of chemistry, who will be the chapter's faculty advisor. She, too, is excited about the proposed diversity lecture series—as a way to inspire and motivate students to finish their programs and pursue STEM careers, and also "showcase our research programs and facilities to diverse early-career scientists and post-doctoral research fellows interested in pursuing independent academic research careers."
"Overall, I think that the University of Rochester community, particularly at the graduate level, will really benefit from having a chapter of the Alliance for Diversity in Engineering and Science on campus," Matson says.
Osborn, who is serving as the chapter's treasurer, does not regret her decision to stay at Rochester to pursue a PhD in translational biomedical science. "I've been very lucky to work with faculty members like Tim Dye, Steve Dewhurst, and Juilee Thakar," she says.
Osborn received a medical center community outreach award as a leader in the Rochester Young Scientists Club's program, which encourages pupils at inner-city elementary schools to start thinking like scientists. She is excited to be serving on the search committee for a new vice president for equity and inclusion at the University.
She is hopeful that ADSE will bring together underrepresented graduate students, now separated by Elmwood Avenue "divide" between the River Campus and the Medical Center and the separate "silos" of their STEM disciplines.
And she agrees with Matson that the University will benefit from having a chapter of ADSE.
"This is an amazing institution, and we have so many resources here. If we can make this a place where people who have different backgrounds feel comfortable, where their different perspectives are welcomed, it can only better the institution as a whole."
23rd WCI Scientific Symposium
Thursday, November 15, 2018
Keynote Lecture in Progress

Supriya Mohile, M.D., M.S.

Judith Campisi, Ph.D.
"This week GDSC assisted the Wilmot Cancer Institute (WCI) in hosting their Twenty Third Scientific Symposium for Cancer Research and Treatment. Graduate Students working in basic, translational and clinical cancer research displayed posters of their respective cancer studies in the Flaum Atrium. GDSC and other faculty gave lectures; including Brian Altman, Stephano Mello, Dirk Bohmann, Vera Gorbunova, Joe Chakkalakal, Laurie Steiner, and Ben Frisch. Additionally, WCI professor Supriya Mohile, gave the Davey Award Lecture titled Improving Care Delivery for Older Patients with Cancer. Finally, Judith Campisi, Ph.D. of the Buck Institute for Research on Aging and USC Leonard Davis School of Gerontology presented the symposium's keynote lecture titled "Cancer and aging: Rival Demons?"
Congratulations Eugene!
Monday, November 12, 2018
We celebrated the successful PhD defense by Eugene Kim last Friday. Working with Jianwen Que, Eugene has identified a significant progenitor cell population in the early foregut. She used a combination of xenopus and mouse models to demonstrate that the transcription factor Isl1 enriched in the unique progenitor population regulates the separation of the esophagus from the trachea. These findings provide important insights into the pathobiology of a relatively common birth defect esophageal atresia with/without trachea-esophageal fistula (EA/TEF). Eugene has a passion for studying developmental biology and stem cells in regeneration, and she plans for a future career in these areas!
Congratulations Fanju!
Thursday, November 8, 2018
On Thursday, Fanju Meng successfully defended his PhD thesis. Under mentorship of Dr. Benoit Biteau, Fanju's studies focus on the regulatory network that coordinates stem cell proliferation and differentiation in the Drosophila intestinal epithelium. Using advanced fly genetics and cell biology methods, Fanju characterized the expression and role of several transcription factors in adult intestinal progenitors. His work significantly improves our understanding of the programs controlling stem cell function and establishes the fruit fly as a model to study these conserved, critical stem cell factors. His findings have been published in Cell Reports and Stem Cell Investigation. And there are additional papers in the pipeline! Fanju was a recipient of a NYSTEM training grant hosted by the Department of Biomedical Genetics, and the Goodman Doctoral Dissertation Fellowship from the University of Rochester. Fanju is now planning on continuing in his work in the field of stem cell and cancer biology using genetic model organisms -- and we wish him the best of luck! You will be missed.
For further reading, please see:
- A Sox transcription factor is a critical regulator of adult stem cell proliferation in the Drosophila intestine. Cell Reports (2015)
- There and back again: amitosis to repopulate a stem cell pool. Stem Cell Investigation (2017)
Fanju Meng Successfully Defending His Ph.D. Thesis
GDSC Halloween Costume Contest!
Thursday, November 8, 2018
Last week GDSC held our Halloween Costume Contest. Our three GDSC student contestants can be seen below.
Derek Crowe earned a very close second place with 14 votes. While first place went to Anne Roskowski with 15 votes. Congrats Anne!"
GDSC Fall Retreat
Thursday, November 1, 2018
Our graduate program in Genetics, Development and Stem Cells (GDSC) celebrated another successful season of research and academic growth. On the afternoon of Friday October 26th 2018, the faculty, students and families of GDSC held our Fall Retreat at the Ellison Park Pavilion Lodge. Among our many reasons to celebrate was our Department's recent faculty expansion including, Brian J. Altman, Stephano Spano Mello, and Patrick J. Murphy. Welcome! We also celebrated the faculty promotion of Benoit Biteau to Associate Professor. Finally, we celebrated the future research of faculty members Margot Mayer-Pröschel, Douglas Portman, Chris Pröschel, and Andy Samuelson each of whom obtained prominent research grants earlier this year. Our festivities included pumpkin carvings, board games and a cocktail hour. There were also three hotly contested rounds of Science Trivia. (The final scores for the first and second place teams were separated by a margin of half a point!) The winning team "Smooth ER" included members Derek Crow, Li Xie, Shen Zhou, Yungeng Pang, Mark Noble, Daxiang Na, and Andy Samuelson. Additionally, Jessie Hogestyn won our "Hidden Facts" contest testing one's knowledge of eccentric or esoteric trivia regarding GDSC faculty and students. Photos of GDSC's genetic festivities can be seen below.
New Research Seeks to Understand Link Between Iron and Brain Development
Wednesday, October 24, 2018
 Iron deficiency is the one of the most prevalent nutritional deficiencies in the world and affects mainly pregnant mothers and young children. Numerous studies have found that mothers who have low iron levels during pregnancy, have a higher risk of giving birth to a child that develops cognitive impairments like autism, attention deficit syndrome, and learning disabilities. While the link between gestational iron deficiency (GID) and cognitive impairment in offspring is well establish, the mechanisms by which this occurs remain unknown.
Iron deficiency is the one of the most prevalent nutritional deficiencies in the world and affects mainly pregnant mothers and young children. Numerous studies have found that mothers who have low iron levels during pregnancy, have a higher risk of giving birth to a child that develops cognitive impairments like autism, attention deficit syndrome, and learning disabilities. While the link between gestational iron deficiency (GID) and cognitive impairment in offspring is well establish, the mechanisms by which this occurs remain unknown.
Margot Mayer-Proschel, Ph.D., an associate professor in the University of Rochester Medical Center (URMC) Department of Biomedical Genetics, has established animal models of GID and was the first to pinpoint the critical periods of gestation during which the developing central nervous system is most vulnerable to GID. Her laboratory recently demonstrated that nerve cells in the brains of animals born to iron deficient mice behave abnormally to excitatory brain stimuli and has shown that functional impairments cannot be restored by iron supplements later in life.
Adrian Moises Molina Vargas is awarded Graduate Alumni Convocation Award
Friday, September 14, 2018
Adrian ('18 University of Alcalá, Spain), one of three new 2018 recruits to the GDSC program was awarded the Graduate Alumni Convocation Award to recognize his promise for exceptional accomplishment in graduate studies. During his year of studying abroad at Tufts during 2017-2018, Adrian worked in the Mirkin lab to study the role of cdc13 mutations in genome instability.
In addition, Sarah Spahr ('18 Ohio State University) was nominated for the Irving Spar Fellowship and Tom O'Connor ('17 University of Buffalo) was nominated for the Newell Stannard Graduate Student Scholarship Award. Congratulations to all three!



Adrian, Sarah & Tom
GDSC Team Participates in 6th annual Wilmot “Warrior Walk”
Friday, September 14, 2018

GDSC Team supports the 2017 Wilmot Cancer Warrior Walk
Students and faculty from Biomedical Genetics and the GDSC program attended the 6th Wilmot Cancer Institute Warrior Walk on Sunday. Aptly named the "NextGen Cancer Busters" to symbolize the graduate students and post-docs training to become cancer researchers, the GDSC team mingled with cancer survivors and family members, to support the fight against cancer. As one team member pointed out: "Meeting cancer survivors really helps put the work in the lab into perspective".
In addition to the Cancer Survivor Walk, "NextGen Cancer Busters" also participated in the 10k and 5k events. Notably, Dalia Ghoneim (5k) and Adam Cornwall (10k) and placed 1st and 2nd in their group, and 2nd and 7th overall. In addition, Scott Friedland and our new faculty addition, Brian Altman, both placed 4th in their age group for the 5k. Congratulations!!
Dr. Mayer-Pröschel to Research the Role of Iron Deficiency in the Developing Brain with a $2 Million Grant
Wednesday, August 29, 2018

Dr. Margot Mayer-Pröschel, Associate Professor in the Department of Biomedical Genetics, with a secondary appointment in the Department of Neuroscience, has received a $2 million, five-year grant to study the impact of gestational iron deficiency (GID) on the development of the brain.
Iron deficiency is still the most prevalent nutritional deficiency in the world. Optimal maternal iron stores during pregnancy are essential for providing adequate iron to the fetal brain. However, many women have insufficient iron reserves to optimally supply the fetus. This is a potentially serious problem as children born to gestational iron deficient (GID) mothers have a higher probability of developing autism, attention deficient syndrome and other cognitive impairments.
A major step towards understanding GID, and potentially preventing multiple types of impairment in children, is to conduct controlled mechanistic and cellular studies in animal models where variable factors can be controlled. Using this approach, the Mayer-Pröschel lab found that low iron supply during pregnancy can cause defects in fetal brain development resulting in a disability to generate appropriate reservoirs of cells that are needed later in life to establish balanced brain activity. This defect persists even if iron supplements are started at birth. This work using a mouse model of GID will provide a detailed understanding of the detrimental effects on child development and may point towards new strategies by which the defects can be normalized or prevented.
The grant, titled Gestational Iron Deficiency disrupts neural patterning in the embryo is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), part of National Institutes of Health.
Dr. Hsu to Continue Stem Cell Research with $2.5 Million Grant
Tuesday, August 21, 2018
Wei Hsu, Ph.D., dean's professor of Biomedical Genetics in the Center for Oral Biology and a scientist with the Eastman Institute for Oral Health at the University of Rochester Medical Center, received a $2.5 million, five year grant to continue his groundbreaking work involving the role of stem cells in tissue regeneration and reconstructive surgical repair.
Currently, the only solution for extensive large bone defects caused by cancer surgery, congenital malformation, trauma and progressive deforming diseases, is to undergo a reconstructive operation. For the 2.2 million cases a year around the world conducted in orthopedic, plastic and oral surgeries, these operations usually involve autografts that require transferring bones from other parts of the body.
"However, bone grafts are encumbered by numerous disadvantages, including donor site morbidity, limited bone supply and complications of extended operating time," Dr. Hsu explained. "The success of such reconstructions also remains highly challenging due to several limitations."
School of Medicine Names New Dean for Graduate Education
Tuesday, August 21, 2018

Richard T. Libby, Ph.D.
Richard T. Libby Ph.D., professor of Ophthalmology and of Biomedical Genetics at the University of Rochester School of Medicine and Dentistry, and a member of the University's Center for Visual Science, has been named Senior Associate Dean for Graduate Education and Postdoctoral Affairs (GEPA), pending approval of the University Board of Trustees. Beginning Sept. 1, Libby will direct the School of Medicine and Dentistry's Ph.D., postdoctoral and master's degree programs. He succeeds Edith M. Lord, Ph.D., who served a decade in the role and is shifting her focus to microbiology and immunology research.
An innovative researcher in the neurobiology of glaucoma, Libby arrived in Rochester in 2006 after postdoctoral and fellowship experiences that enlightened him on the power of model genetics systems in the study of eye disease. Years spent training at the Medical Research Council's Institute for Hearing Research in Nottingham, England, and the Jackson Laboratory in Bar Harbor, Maine, formed the foundation for his current laboratory, which is focused on understanding the cell signaling pathways that lead to vision loss in glaucoma.
Libby is director of the Cell Biology of Disease Graduate Program, has served on numerous academic committees integral to research activities and graduate education, and is a respected mentor and teacher. He has published, as author or co-author, more than 60 peer-reviewed scientific articles and numerous reviews, book chapters and commentaries, and has presented internationally on a range of topics in eye and vision research.
"Rick understands that excellence in a research enterprise is essential to attracting the best and brightest talent and has articulated a vision for further improving the experience here, making it clear to the outside world that Rochester is the best place to learn and study," said Mark Taubman, M.D., CEO of the Medical Center and Dean of the School of Medicine and Dentistry at the University of Rochester. "He is a passionate scientist whose experience in a clinical department will bring valuable insight to graduate programs in basic and clinical research—a true asset to his role in helping prepare future generations of scientists."
"Complementing his expertise in leading graduate programs, and thorough understanding of their needs, Rick has developed a thoughtful approach to what it will take to continue moving them forward. It's clear that he's driven by a desire to develop our trainees and motivated to give them the best graduate/postdoctoral experience possible," said Stephen Dewhurst, Ph.D., Vice Dean for Research at the School of Medicine and Dentistry and Associate Vice President for Health Sciences Research at the University of Rochester. "In addition, having developed his own career in a somewhat untraditional way, Rick brings an added dimension to understanding and supporting others who are exploring diverse career options."
Libby received a doctorate degree in biology from Boston College in the field of neurodevelopment. He was a postdoctoral fellow at the Medical Research Council's Institute for Hearing Research in Nottingham England, and a postdoctoral fellow at the Jackson Laboratory in Bar Harbor, Maine. He joined the School of Medicine and Dentistry faculty as an assistant professor in 2006, was named associate professor in 2012, and professor in 2018.
"Rick is a great choice to succeed Edith Lord as the Senior Associate Dean for Graduate Education," said Dirk Bohmann, Ph.D., Donald M. Foster, M.D. Professor of Biomedical Genetics and Senior Associate Dean for Basic Research, who led the search committee. "He realizes that research excellence and successful graduate and postdoctoral programs are mutually dependent. You cannot have one without the other. He will be a passionate advocate for the graduate students and postdocs."
"Under Dr. Lord's leadership, GEPA has greatly enhanced the support and training of URMC's graduate students and postdoctoral fellows," Libby said. "In fact, GEPA has helped lead the nation in providing enhanced educational opportunities to ready trainees for the numerous careers available to the modern-day scientist. I am excited to be a part of this team. I look forward to further developing GEPA's missions of providing world-class training for our graduate students and postdoctoral fellows, and to helping our trainees continue their important work focused on understanding human health and disease."
Lord's four-decade career in Rochester is dotted with milestones and accomplishments. She joined the School of Medicine and Dentistry faculty as a senior instructor in 1976 and rose through the ranks to professor in 1994. In 10 years as Senior Associate Dean, she worked to improve the experience of graduate students and postdocs in and outside the lab, adding Postdoctoral Affairs to the Office for Graduate Education's name, standardizing salaries and benefits, and advocating on behalf of trainees. She spearheaded a revamping of the fundamental basic science courses, incorporating more workshops and active learning components and emphasizing team-based science. She also fostered professional development initiatives and guided efforts to support students' health and wellbeing. Her return to the research lab will include focusing on an NIH grant to study the immune response in tumors.
Hucky Land Leads Genome Sequencing Project that Expands Tissue-Banking Partnership
Thursday, August 2, 2018
URMC recently extended a previous tissue-banking agreement with Indivumed, a private company, to include a new partnership for whole genome sequencing. Hucky Land, Ph.D., director of research at the Wilmot Cancer Institute, is leading the partnership with a project to sequence 2,000 colorectal cancer samples from 1,000 patients by the end of the year, and to develop novel software tools enabling complex cancer data analysis in the future.
The latest agreement with Indivumed also enabled the URMC Genomics Research Center (GRC) to purchase a state-of-the-art Illumina NovaSeq6000 DNA sequencer. The equipment signals a new era for genomics at the University, as it will aid research advancing personalized medicine, including discovery of new therapies to improve cancer outcomes. It also allows URMC investigators to perform whole genome sequencing for a fraction of the previous cost.
Based in Germany, Indivumed helped the URMC in 2016 set up a centralized tissue bank for collecting high-quality samples from surgical patients for cancer research. Once a tissue sample is examined to make a diagnosis, the remaining tissue is stored in the "Rochester Cancer Library" and available to URMC, Wilmot, and Indivumed scientists. The samples can be correlated with patient survival data, response to treatment, and other meaningful information for researchers.
In addition to Land, faculty who have been integral to the agreements with Indivumed include Steve Dewhurst, Ph.D., Vice Dean for Research at the SMD; David Linehan, M.D., chair of Surgery, director of Clinical Operations at Wilmot, and a pancreatic cancer researcher, who serves as the supervising investigator for tissue banking; and Bruce Smoller, M.D., chair of Pathology and Laboratory Medicine, who led efforts to operationalize the tissue bank.
Stephano Mello lab to open
Tuesday, July 10, 2018
Stephano is a currently a Postdoctoral fellow at Stanford University, and will join the faculty in the Department of Biomedical Genetics at the University of Rochester, in September 2018. Stephano is coming from Dr. Laura Attardi’s lab and his research seeks to understand the mechanisms behind pancreatic cancer initiation.
Please visit his lab site for more information.
Wilmot announces new Pilot Award recipients
Monday, April 30, 2018
Wilmot's competitive seed-grant program aims to fund research projects that will generate preliminary data necessary to potentially apply for federal funding in the future. Thanks to financial support from two community organizations -- Adding Candles for a Cure and the Edelman Gardner Cancer Research Foundation -- four projects have received funding that started Jan. 1.
Mark Noble, Ph.D., Professor in the departments of Biomedical Genetics and Neuroscience, received a $50,000 grant for his project titled, "A biomarker for a novel glioblastoma (GBM) vulnerability." The co-investigators for this project are Kevin Walter, M.D., Mahlon Johnson, M.D., Ph.D., Nimish Mohile, M.D., and Peggy Auinger, M.S.
Bradford Mahon, Ph.D., Assistant Professor in the Departments of Neurology and Neurosurgery, received a $50,000 grant for his project seeking to demonstrate feasibility and preliminary efficacy of advanced MRI mapping in improving outcome in patients with glioblastoma. Kevin Walter, M.D., is the co-investigator for this project.
Congratulations to all Wilmot pilot grant recipients.
Pilot Funding In Stem Cell And Regenerative Medicine Research
Friday, April 20, 2018
The University of Rochester Stem Cell and Regenerative Medicine Institute invites applications for Strategic Plan Pilot Grants in Stem Cell Research
Read Full Application for Strategic Plan Pilot Grants in Stem Cell Research
Pilot Funding On Novel Treatment Of Chronic Spinal Cord Injury
Friday, April 20, 2018
The University of Rochester Spinal Cord Injury Research Program invites applications for a pilot grant application on treatment of chronic spinal cord injury.
Read Full Application for pilot of treatment of chronic spinal cord injury
Biological Sex Tweaks Nervous System Networks, Plays Role in Shaping Behavior
Thursday, March 8, 2018
By Mark Michaud
New research published today in the journal Current Biology demonstrates how biological sex can modify communication between nerve cells and generate different responses in males and females to the same stimulus. The findings could new shed light on the genetic underpinnings of sex differences in neural development, behavior, and susceptibility to diseases.
"While the nervous systems of males and females are virtually identical, we know that there is a sex bias in how many neurological diseases manifest themselves, that biological sex can influence behavior in animals, and that some of these differences are likely to be biologically driven," said Douglas Portman, Ph.D., an associate professor in the Departments of Biomedical Genetics, Neuroscience, and the Center for Neurotherapeutics Discovery at the University of Rochester Medical Center (URMC) and lead author of the study. "This study demonstrates a connection between biological sex and the control and function of neural circuits and that these different sex-dependent configurations can modify behavior."
The findings were made in experiments involving the nematode C. elegans, a microscopic roundworm that has long been used by researchers to understand fundamental mechanisms in biology. Many of the discoveries made using these worms apply throughout the animal kingdom and this research has led to a broader understanding of human biology. In fact, three Nobel Prizes in medicine and chemistry have been awarded for discoveries involving C. elegans.
The study focuses on the different behaviors of male and female worms. There are two sexes of C. elegans, males and hermaphrodites. Although the hermaphrodites are able to self-fertilize, they are also mating partners for males, and are considered to be modified females.
The behavior of C. elegans is driven by sensory cues, primarily smell and taste, which are used by the worms to navigate their environment and communicate with each other. Female worms secrete a pheromone that is known to attract males who are drawn by this signal in search of a mate. Other females, however, are repelled by the same pheromone. It is not entirely understood why, but scientists speculate that that the pheromone signals to females to avoid areas where there may be too much competition.
Leader in the field of epigenetic regulation and cancer biology joins the Department of Biomedical Genetics and GDSC Program
Wednesday, March 7, 2018
 Dr. Paula Vertino, currently the leader of the Cancer Genetics and
Epigenetics Program at Emory University will be joining the University of Rochester Department in Biomedical
Genetics and the Wilmot Cancer Institute this summer. Dr. Vertino's research on cancer epigenetics will greatly
expand our areas of research strengths. She is an exceptionally important player in her field, and we look forward
to welcoming her to the GDSC program!
Dr. Paula Vertino, currently the leader of the Cancer Genetics and
Epigenetics Program at Emory University will be joining the University of Rochester Department in Biomedical
Genetics and the Wilmot Cancer Institute this summer. Dr. Vertino's research on cancer epigenetics will greatly
expand our areas of research strengths. She is an exceptionally important player in her field, and we look forward
to welcoming her to the GDSC program!
Congratulations to our newly minted PhD’s!
Wednesday, February 28, 2018
Michelle Warren Millar
Through the course of her studies on melanoma, Michelle has found that GPR56 inhibits metastatic growth by preventing the expansion of micrometastases into macrometastases. Loss of GPR56 in highly metastatic cells promotes cell-ECM signaling in these metastatic lesions, and inhibition of this signaling results in smaller, less proliferative metastases, targeting one of the deadliest, metastasizing tumor types.
 Yang Cheng
Yang Cheng
Yang's research focuses on mechanisms that drive aging. Using Drosophila as a model, she showed that age-associated changes in chromatin structure explain why older organisms become more vulnerable to oxidative damage and decline functionally. Based on these findings she has developed strategies that can slow down epigenetic aging and improve the fitness of old organisms. This research raises intriguing implications for healthy aging in humans.
A Noble pursuit; finding the best science to help the most people
Tuesday, February 20, 2018
By Kevin McCormack

Mark Noble. Photo by Todd Dubnicoff
Mark Noble, Ph.D., is a pioneer in stem cell research and the Director of the University of Rochester Stem Cell and Regenerative Medicine Institute in New York. He is also a member of CIRM’s Grants Working Group (GWG), the panel of independent scientific experts we use to review research applications for funding and decide which are the most promising.
Mark has been a part of the GWG since 2011. When asked how he came to join the GWG he joked: “I saw an ad on Craigslist and thought it sounded fun.” But he is not joking when he says it is a labor of love.
“My view is that CIRM is one of the greatest experiments in how to develop a new branch of science and medicine. If you look at ventures, like the establishment of the National Institutes of Health, what you see is that when there is a concentrated effort to achieve an enormous goal, amazing things can happen. And if your goal is to create a new field of medicine you have to take a truly expansive view.”
Mark has been on many other review panels but says they don’t compare to CIRM’s.
“These are the most exciting review panels in which I take part. I don’t know of any comparable panels that bring together experts working across such a wide range of disciplines and diseases. It’s particularly interesting to be involved in reviews at this stage because we get to look at the fruits of CIRM’s long investment, and at projects that are now in, or well on the way towards, clinical trials.
It’s a wonderful scientific education because you come to these meetings and someone is submitting an application on diabetes and someone else has submitted an application on repairing the damage to the heart or spinal cord injury or they have a device that will allow you to transplant cells better. There are people in the room that are able to talk knowledgeably about each of these areas and understand how the proposed project might work in terms of actual financial development, and how it might work in the corporate sphere and how it fits in to unmet medical needs. I don’t know of any comparable review panels like this that have such a broad remit and bring together such a breadth of expertise. Every review panel you come to you are getting a scientific education on all these different areas, which is great.”
Another aspect of CIRM’s work that Mark admires is its ability to look past the financial aspects of research, to focus on the bigger goal:
“I like that CIRM recognizes the larger problem, that a therapy that is curative but costs a million dollars a patient is not going to be implemented worldwide. Well, CIRM is not here to make money. CIRM is here to find cures for unmet medical needs, which means that if someone comes in with a great application on a drug that is going to cure some awful disease and it’s not going to be worth a fortune, that is not the main concern. The main concern is that you might be able to cure this disease and yeah, we’ll put up money to help you so that you might be able to get into clinical trials, to get enough information to find out if it works. And to have the vision to go all the way from, ‘ok, you guys, we want you to enter this field, we want you to be interested in therapeutic development, we are going to help you structure the clinical trials, we are going to provide all the Alpha Stem Cell Clinics that can talk to each other to make the clinical trials happen.
The goal of CIRM is to change medicine and these are the approaches that have worked really well in doing this. The CIRM view clearly is:
‘There are 100 horses in this race and every single one that crosses the finish line is a success story.’ That’s what is necessary, because there are so many diseases and injuries for which new approaches are needed.”
Mark says working with CIRM has helped him spread the word back home in New York state:
“I have been very involved in working with the New York state legislature over the years to promote funding for stem cell biology and spinal cord injury research so having the CIRM experience has really helped me to understand what it is that another place can try and accomplish. A lot of the ideas that have been worked out at CIRM have been extremely helpful for statewide scientific enterprises in New York, where we have had people involved in different areas of the state effort talk to people at CIRM to find out what best practice is.”
Mark says he feels as if he has a front row seat to history.
“Seeing the stem cell field grow to its present stage and enhancing the opportunity to address multiple unmet medical needs, is a thrilling adventure. Working with CIRM to help create a better future is a privilege.”
Heard of the Microbiome? Meet the MicroRNAome.
Tuesday, October 31, 2017
By Susanne Pallo
A University of Rochester Medical Center researcher is part of a team that is working toward characterizing where a certain type of RNA, called microRNA, is expressed in human cells. In a recent study, published in Genome Research, the team made a giant set of data about these RNA available to the public to guide research and foster the development of new therapies.
RNA, the close cousin of DNA, comes in many flavors - each with a specific role. MicroRNA (miRNA) help regulate which proteins are produced in a cell and how much. Fiddling with the level of proteins can have subtle or large impacts on that cell's activity and can even cause disease.
Several studies have linked miRNAs to diseases, either as the cause or simply a marker. While some of these links could lead to new treatments, others may just be red herrings.
"We've showed previously that when somebody claims a certain miRNA is a marker of a disease, at times it's just a marker of increased inflammation, which is a side effect of a lot of diseases," said Matthew N. McCall, Ph.D., assistant professor of Biostatistics and Biomedical Genetics at URMC and an author on the study.
According to McCall and study leader Marc K. Halushka, M.D., Ph.D., associate professor of Pathology and director of Oncology Tissue Services at Johns Hopkins University School of Medicine, before you can understand what miRNAs are doing, you need to know where they are.
And why is that important?
Within a given tissue in your body, there could be thousands of different cells types, serving different purposes. When measuring RNA in a tissue biopsy, you will get a mixture of RNA from all of these different cell types, whether or not they are relevant to the disease you are interested in.
For their study, Halushka's team pulled together all that was known about miRNA in human cells and conducted experiments to fill the gaps. Though it wasn't their specific intent, the researchers found that many miRNAs originally thought to be expressed in all or many cell types actually are not. Rather, they are expressed in cells that get into all or most tissues, like blood or inflammatory cells, and could be mistakenly associated with a disease.
All of the data from Halushka's study are available in an online database and in the University of California, Santa Cruz Genome Browser and will be updated regularly. Having access to this data should save researchers a lot of time and help weed out some those aforementioned red herrings. With just a few clicks, researchers can find all of the cells types that express a specific miRNA or all of the miRNAs expressed in a specific cell type.
“Bubbles” Boost Search for Treatment to Aid Head and Neck Cancer Patients
Wednesday, October 25, 2017

Catherine Ovitt, Danielle Benoit, and Lisa DeLouise
A scientific team at the University of Rochester is using innovative technology to discover preventative treatments for salivary gland radiation damage typical for head and neck cancer patients—and recently received a $3.8 million National Institutes of Health grant to support their investigation.
Cancer patients can lose salivary gland function during treatment for head and neck tumors. The irreversible damage, which prevents patients from producing saliva, often results in permanent dry mouth and makes it difficult to eat, speak, and swallow. The team will develop salivary gland tissues using a unique chip technology called "microbubbles," which are tiny spherical wells or bubbles that can hold cells.
The use of the microbubble platform is based on several years of salivary gland research, led by Catherine E. Ovitt, Ph.D., associate professor of Biomedical Genetics, a member of the UR Center for Oral Biology, and an expert in the repair and regeneration of salivary glands, and Danielle Benoit, Ph.D., associate professor of Biomedical Engineering and an expert in drug delivery systems and hydrogel platforms for tissue engineering approaches. Together with Lisa A. DeLouise, Ph.D., associate professor of Dermatology and Biomedical Engineering, who developed and received several patents for the microbubble concept, the scientists are working as co-principal investigators on the NIH project.
Their goal is to find drugs that could be given to patients prior to radiation treatment that would prevent damage to the glands.
"Dr. Ovitt and I have shown through years of investigation that being able to develop functional salivary gland tissue for testing is the key to solving this problem," Benoit said. "So, it's microbubbles to the rescue."
Expanding cells and tissue outside of the body is elusive. In this case the process involves taking salivary gland cells that have been removed from humans undergoing surgery, expanding the cells, and studying their reaction to various drugs.
A major problem, however, starts to occur as soon as the tissue is removed from the body and isolated: Cells immediately begin to lose their natural function. In the body, cells send signals and secrete proteins that are essential for their survival. In a culture plate in a laboratory, however, these signals and proteins are diluted and dispersed, making the cells no longer viable.
DeLouise's technology at first glance looks similar to a cell culture petri dish, a round piece of silicone about the size of the large cookie. But within the dish are an arrangement of thousands of tiny round "micro-wells," each one comprising a minuscule compartment for cell growth and tissue formation. The unique shape of each microbubble creates a niche that concentrates the cells, allowing them to proliferate and form salivary gland units.
The microbubbles come in different sizes, and the beauty of the technology is that scientists can grow cells in thousands of bubbles at one time. DeLouise can make dishes the size of a dime that include more than 5,000 microbubbles. In addition, Benoit's lab has produced hydrogel materials that can be placed inside each microbubble that further allow the cell to maintain its structure and function.
If the team can successfully grow human salivary gland cells in the microbubbles, they say, they will also be able to rapidly test thousands of existing Food and Drug Administration-approved drugs on the salivary tissue using the microbubble technology.
"Only one treatment is currently available for radioprotection but it comes with many side effects, so most patients discontinue it," Ovitt said. "There is a great need for additional ways to either cure or prevent this debilitating condition."
The team is collaborating with Shawn D. Newlands, M.D., Ph.D., M.B.A., chair of the Department of Otolaryngology and member of the Wilmot Cancer Institute's head and neck oncology team, to collect salivary tissue from consenting patients undergoing salivary gland surgery. Salivary gland cells are isolated from these tissues for seeding into microbubbles for the investigation. Additionally, Paul Dunman, Ph.D., associate professor of Microbiology and Immunology, will provide high-throughput drug-screening expertise during the second phase of the project, which is contingent upon successful development of the human gland chips.
GDSC Team supports the 2017 Wilmot Cancer Warrior Walk
Sunday, September 10, 2017
Several GDSC students and faculty attended the 5th Wilmot Cancer Warrior Walk this Sunday. Showing off our colors in form of this year's new GDSC T-shirts, the Team participated very successfully in the 10K, 5K and 1M events. Adam Cornwell, Andrew Albee, Xiaolu Wei, Fanju Meng, Justine Melo and Dalia Ghoneim were our "Runing Warriors" for the 10k and 5k events, with Andrew Allbee finishing overall 6th (44:05 min, 3rd 20-29yr old) and Adam Cornwell 7th (44:40 min, 1st 30-39yr old) in the 10k. Dalia Ghoneim ran the 5k and was the 3rd overall female finisher, and was 2nd in her age category (30-39 years). Her time was 23:33. Congratulations to all! The team was rounded out by Dashiell Na, Shen Zhou and and Anne Roskowski. Led by the scientific director of the Wilmot Cancer Center, Hucky Land, faculty also attended in force, including the whole Samuelson family, cancer biologist Mark Noble and the Pröschel's. Runners and walkers alike enjoyed a beautiful sunny day out and the positively uplifting company of cancer fighters and survivors! See you all again in 2018!
Facebook Post
GDSC Picnic Kicks-Off the 2017-2018 Academic Year
Thursday, September 7, 2017
Braving at times inclement weather, Graduates students and Faculty of the Genetics, Development and Stem Cell program gathered at he Genessee Valley Park hawthorne lodge for food, fun and festivities. After a week of rain and clouds from the outer bands of Hurricane Harvey, we caught a break and ended the day with sunshine! A fitting start to the new academic year and a warm welcome to Anne Roskowski, a new graduate student in the GDSC program.
Doctors Might FINALLY Be Able to Tell If Your Infection Is Bacterial or Viral
Monday, August 14, 2017
If you head to your doctor with a fever and cough or other signs you're getting sick, there's a good chance your doctor won't know what's behind it. It's hard to test for certain diseases, like bacterial pneumonia.
When patients come in with respiratory viruses like the common cold and sinus infections, doctors often send them home with an antibiotic prescription. The problem is, antibiotics only target bacteria and won't do anything to fight a virus. Still, about 30 percent of antibiotics prescriptions are for viral infections, according to the CDC. (Make sure to ask these essential questions before taking antibiotics.)
Not only is an antibiotic completely unhelpful against a virus, but it could have major consequences. Antibiotics kill most of the bacteria behind your infection—but not all of them. The bacteria that are strong enough to survive will multiply to create more bacteria that are resistant to treatment, too. Over time, this means there will be more resistant bacteria than ones that antibiotics can kill, so the infections will be harder to treat.
And if you take antibiotics when you don't need to, or use antibacterial soap, you're speeding that process along. Eventually, diseases that used to be easy to treat with antibiotics could become dangerous again.
Luckily, researchers might have found a way to tell the difference between bacterial and viral infections, so you won't get a useless antibiotic. In a study in the journal Scientific Reports, researchers took blood from 94 patients who had lower respiratory tract infections. Lab tests found genetic markers in the blood that could correctly figure out if an infection was viral or bacterial 80 to 90 percent of the time. (Find out how genetic tests could help you lose weight, too.)
Because the sample size was so small, more tests will be needed before doctors can start using this diagnosis method in their offices. But if it does take off, it will be a lot easier than testing for specific diseases.
"Our genes react differently to a virus than they do to bacteria," says study co-author Thomas Mariani, PhD, pediatrics, environmental medicine, and biomedical genetics professor at University of Rochester Medical Center, in a statement. "Rather than trying to detect the specific organism that's making an individual sick, we're using genetic data to help us determine what's affecting the patient and when an antibiotic is appropriate or not."
In the meantime, if you think you're not feeling well, use these natural remedies for cold and flu.
Congratulations Margaret!
Wednesday, July 26, 2017

Margaret Hill
On Monday PhD candidate Margaret Hill presented her work investigating intrahepatic cholangiocarcinoma (iCCA), a form of liver cancer which morphologically resembles the biliary tract. Margaret completed her work under guidance of Dr. Aram Hezel. Her work helps us to understand the interplay between chronic liver injury, a common risk factor for this cancer and the cell of origin as she proved that hepatocytes, as opposed to biliary cells, may serve as a cell of origin for this cancer. Further investigation into important pathways known to be activated in biliary-derived iCCA showed hepatocyte-derived iCCA similarly up-regulates the Wnt and Notch pathways and thus could be targeted for treatment. Margaret went on to probe the importance of MCL-1, the most commonly amplified gene in iCCA, and identified a genetic subset of iCCA cancers which appear to depend on MCL-1 expression. Together, Margaret's work may have important therapeutic implications for iCCA. Well done Margaret and congratulations to Aram!
Hidden Herpes Virus May Play Key Role in MS, Other Brain Disorders
Monday, July 10, 2017
The ubiquitous human herpesvirus 6 (HHV-6) may play a critical role in impeding the brain’s ability to repair itself in diseases like multiple sclerosis. The findings, which appear in the journal Scientific Reports, may help explain the differences in severity in symptoms that many people with the disease experience.
“While latent HHV-6 – which can be found in cells throughout the brain – has been associated with demyelinating disorders like multiple sclerosis it has not been clear what role, if any, it plays in these diseases,” said Margot Mayer-Proschel, Ph.D., an associate professor at the University of Rochester Medical Center Department of Biomedical Genetics and co-author of the study. “These findings show that, while in the process of hiding from the immune system, the virus produces a protein that has the potential to impair the normal ability of cells in the brain to repair damaged myelin.”
Stem Cells May Be the Key to Staying Strong in Old Age
Tuesday, June 13, 2017
University of Rochester Medical Center researchers have discovered that loss of muscle stem cells is the main driving force behind muscle decline in old age in mice. Their finding challenges the current prevailing theory that age-related muscle decline is primarily caused by loss of motor neurons. Study authors hope to develop a drug or therapy that can slow muscle stem cell loss and muscle decline in the future.
Video of 3 Minute Thesis Event
Thursday, June 8, 2017
We have the video of the full event with all presentations fully captions and with the slides running in time with the videos.
3MT Presenters, Programs & Topics
Thesis presentations in order
- Stephanie Carpenter (Chemistry) - Solving the Mystery of Iron Chemistry
- Sarah Catheline (Pathways of Human Disease) - Inhibiting Inflammaging to Treat Osteoarthritis(OA)
- Scott Friedland (Genetics, Development & Stem Cells) - Pancreatic Cancer and the Tale of the Broken Librarian
- Claire McCarthy (Toxicology) - Investigating the Toxicological Effects of Dung Biomass Smoke Exposure
- Taylor Moon (Immunology, Microbiology and Virology) - The New Epidemic
- Thuy-Vy Nguyen (Social-Personality Psychology) - Solitude *Winner*
- Manisha Taya (Cellular & Molecular Pharmacology and Physiology) - Understanding Lymphangioleiomyomatosis (LAM): The “Other” Steroid-Dependent Cancer From Bed-Side to Bench and Back Again
- Janelle Veazey (Immunology, Microbiology and Virology) - Role of Protein Kinase D in Epithelial Cells During Respiratory Infection
Scott Friedland takes 2nd place in the Three Minute Thesis (3MT) competition
Monday, May 15, 2017

On May 11th, 2017, Scott Friedland took 2nd place in the Three Minute Thesis (3MT) competition with his talk entitled, “Pancreatic Cancer and the Tale of the Broken Librarian. 3MT, created at The University of Queensland in Australia, is an effort to bring awareness to research and scientific communication, in which competitors have 3 minutes to get across the thrust of their thesis to a general audience. Scott is an MD/PhD student currently working in the lab of Dr. Aram Hezel in the Genetics, Development, and Stem Cells program. His research focuses on defining the role of ARID1A and the SWI/SNF complex in pancreatic cancer and development.
29th Annual Genetics Day Symposium
Thursday, May 4, 2017
This year's Genetics Day provided another opportunity to celebrate the impact of Genetics on science and medicine. The program consisted of four short talks by U of R scientists, a poster session and the Keynote event, the Fred Sherman Lecture, delivered this year by Dr. David Sabatini from the Whitehead Institute for Biomedical Research at MIT. Dr. Sabatini talked about “Growth Regulation by the mTOR Pathway”.
This year’s Genetics Day Poster Session included posters from research laboratories across the University. Three graduate students and one post-doctoral associate were awarded poster prizes:

Andrew Allbee – Biteau Lab
dLMX1A is Required for Drosophila Ovary Stem Cell-Niche Unit Establishment
Amber Cutter – Hayes Lab
Molecular Characterization of Nucleosome Recognition by Linker Histone H1.0
Browyn Lucas – Maquat Lab
Evidence for Convergent Evolution of Sines for Staufen-Mediated Control of MRNA Decay
Janet Lighthouse – Small Lab
Expression Profiling Reveals the Cardioprototective Role of Metallothioneins in Exercise
Genetics Day has been a long-standing tradition at the University of Rochester and more recently includes the Fred Sherman lecture in memory of Fred Sherman a renowned biochemist and geneticist, who led international efforts to establish the yeast Saccharomyces cerevisiae as the premier genetic eukaryotic model system. The lecture is made possible by a generous fund endowed by Fred Sherman's wife, Elena Rustchenko-Bulgac, herself a research professor at the URMC.
GDSC Graduate Justin Komisarof successfully defends Friday April 28
Tuesday, May 2, 2017
MD/PhD candidate Justin Komisarof presented his work on the hunt for a common cancer gene signature. Building on prior work by the Land and McMurray labs, Justin used bioinformatic approaches to search for the presence of a ‘cooperation response gene’ (CRG) cancer gene expression signature a broad panel of different tumors. Using microarray gene expression datasets from GEO in colon, pancreatic, prostate, and head and neck cancers, Justin found a core set of consistently disregulated CRGs. A more in-depth study of genotype-phenotype correlation in colon cancer samples revealed that this CRG dysregulation did not track with p53 or Ras mutations, suggesting that CRG dysregulation occurs in multiple human cancers, and that the CRG expression pattern is independent of mutational status and may emerges as a function of the malignant state. Justin also applied this CRG-based analysis to prostate cancer samples and identified a core set of CRGs that when combined with clinical staging predicts recurrent outcomes with greater than 80% accuracy – a highly significant improvement over current methods. Justin’s work provides an important step forward for both diagnostic and future therapeutic approaches.
A four gene signature predictive of recurrent prostate cancer. Komisarof JMcCall M, Newman L, Bshara W, Mohler JL, Morrison C, Land H. Oncotarget. 2017 Jan 10;8(2):3430-3440.
GDSC Students attend the March for Science
Tuesday, April 25, 2017
Students from the Genetics Program attended The Rochester March for Science on Saturday April 22

Fanju Meng (Biteau Lab), Sreejith (Biteau Lab), Emily Wexler (Portman Lab),
Sebastian Rojas Villa (Biteau Lab), Robert Hoff (Bohmann Lab), Andrew Allbee (Biteau Lab)
Michael John Beltejar awarded CTSI Pilot Trainee Grant
Thursday, April 13, 2017
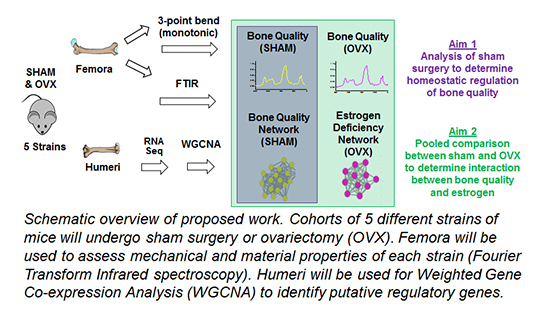
Michael John Beltejar, a 4th year student in the Genetics, Development, and Stem Cells PhD program with a research focus in Genetic contributions to bone strength has been awarded CTSI Pilot Training Grant.
CTSI Pilot Trainee Grant RFA
A major priority for the CTSI is the active support of research collaborations via cross-disciplinary collaboration, and the support of research that addresses significant problems related to population health. Thus, applications directly addressing these areas are strongly encouraged. Trainee awards help awardees obtain the most prestigious fellowship possible following the project. The project should be part of a long-term plan to become an independent investigator. The award provides a maximum of $25,000 for a period of one year.
Project Description
Osteoporosis remains a significant concern. By 2025, osteoporotic fractures are expected to increase to 3 million with an estimated cost of $25.3 billion. Morbidity and mortality increases following all major fractures in patients over 55 years of age. It is widely understood that compromised bone strength is the underlying pathophysiology of osteoporosis. Currently, bone mineral density (BMD), is the basis for diagnosis and the main target for osteoporosis therapy. BMD is an important clinical tool, but explains only 55 % of fractures, leaving the needs of 45% of patients unaddressed. Fundamentally, bone strength has two interconnected but distinct components: quantity and quality. While BMD reflects quantity, bone quality (BQ) reflects morphologic and compositional properties. However, developing therapies based on BQ is limited by two major gaps in knowledge: 1) Which genes regulate BQ and 2) Does estrogen deficiency modify the genetic regulation of BQ? Therefore, the objective of the work proposed is to construct a genetic network to identify candidate genes regulating bone composition(SA1) and determine if estrogen deficiency interacts with these genes(SA2).
The rationale for the proposed work is that bridging these gaps in knowledge is a crucial first step in the identification of novel therapeutics. The results produced from SA1 will inform ongoing research in the lab--in particular, a genome-wide association study identifying locations in the genome that are responsible for bone quality. Secondly, the results of SA2 have a strong potential to unlock research on multiple levels osteoporosis management. Identification of novel genes could be used as a new biomarker to identify at risk individuals earlier. Downstream activity of these genes could become additional metrics to monitor disease progression. Finally, these genes become putative targets for novel therapies. The research in this application is innovative because it is a significant departure which shifts focus from BMD to BQ as an equally important contributor of fracture resistance.